Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Furuike, Yoshihiko*; Ouyang, D.*; Tominaga, Taiki*; Matsuo, Tatsuhito*; Mukaiyama, Atsushi*; Kawakita, Yukinobu; Fujiwara, Satoru*; Akiyama, Shuji*
Communications Physics (Internet), 5(1), p.75_1 - 75_12, 2022/04
Times Cited Count:4 Percentile:65.23(Physics, Multidisciplinary) glycoprotein; Microbial transformation of heavy elements in the aquatic environment
glycoprotein; Microbial transformation of heavy elements in the aquatic environmentKozai, Naofumi; Sakamoto, Fuminori; Tanaka, Kazuya; Onuki, Toshihiko; Sato, Takahiro*; Kamiya, Tomihiro*; Grambow, B.
Chemosphere, 196, p.135 - 144, 2018/04
Times Cited Count:5 Percentile:17.39(Environmental Sciences)Transformation of heavy elements by microbes such as bacteria and fungi has been an intense research subject; however, little is known about that of protozoa. This study investigated interaction of a representative protozoa,  , with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living
, with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living  cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
Unno, Masayoshi*; Sugishima, Masakazu*; Wada, Kei*; Hagiwara, Yoshinori*; Kusaka, Katsuhiro*; Tamada, Taro; Fukuyama, Keiichi*
Nihon Kessho Gakkai-Shi, 57(5), p.297 - 303, 2015/10
Bilin compounds are fundamentally important for oxygenic photosynthetic organisms, because they are utilized as pigments for photosynthesis (phycobilins) and photoreceptors (phytochromobilin). Phycocyanobilin (PCB), a phycobilin, comprises the chromophore of algal phytochromes and the core phycobiliprotein antennae of cyanobacteria and red algae. PCB is biosynthesized by a member of the ferredoxin-dependent bilin reductase family, phycocyanobilin:ferredoxin oxidoreductase (PcyA). In the present study, we determined the neutron crystal structure of PcyA in complex with its substrate biliverdin (BV). This neutron structure revealed the protonation state of BV and the surrounding residues. We found that two forms of BV, neutral BV and protonated BVH , were coupled with the two conformation/protonation states of the essential residue Asp105. Further, His88 and His74 near BV were singly protonated and were connected with an intervening hydronium ion. Neutron analysis also revealed how X-ray irradiation of the PcyA-BV crystal altered the structure of the PcyA-BV complex.
, were coupled with the two conformation/protonation states of the essential residue Asp105. Further, His88 and His74 near BV were singly protonated and were connected with an intervening hydronium ion. Neutron analysis also revealed how X-ray irradiation of the PcyA-BV crystal altered the structure of the PcyA-BV complex.
Omichi, Masaaki*; Asano, Atsushi*; Tsukuda, Satoshi*; Takano, Katsuyoshi*; Sugimoto, Masaki; Saeki, Akinori*; Sakamaki, Daisuke*; Onoda, Akira*; Hayashi, Takashi*; Seki, Shu*
Nature Communications (Internet), 5, p.3718_1 - 3718_8, 2014/04
Times Cited Count:35 Percentile:77.9(Multidisciplinary Sciences)Protein nanowires exhibiting specific biological activities hold promise for interacting with living cells and controlling and predicting biological responses such as apoptosis, endocytosis and cell adhesion. Here we report the result of the interaction of a single high-energy charged particle with protein molecules. Degradation of the human serum albumin nanowires was examined using trypsin. The biotinylated human serum albumin nanowires bound avidin, demonstrating the high affinity of the nanowires. Human serum albumin-avidin hybrid nanowires were also fabricated from a solid state mixture and exhibited good mechanical strength. The biotinylated human serum albumin nanowires can be transformed into nanowires exhibiting a biological function such as avidin-biotinyl interactions and peroxidase activity. The present technique is a versatile platform for functionalizing the surface of any protein molecule with an extremely large surface area.
Jochi, Yasumasa*; Kitao, Akio*; Go, Nobuhiro
Journal of the American Chemical Society, 127(24), p.8705 - 8709, 2005/06
Times Cited Count:27 Percentile:61.14(Chemistry, Multidisciplinary)no abstracts in English
Basu, G.*; Sivanesan, D.*; Kawabata, Takeshi*; Go, Nobuhiro
Journal of Molecular Biology, 342(3), p.1053 - 1066, 2004/09
Times Cited Count:23 Percentile:35.48(Biochemistry & Molecular Biology)no abstracts in English
Niimura, Nobuo; Chatake, Toshiyuki; Kurihara, Kazuo; Maeda, Mitsuru
Cell Biochemistry and Biophysics, 40(3), p.351 - 369, 2004/06
Times Cited Count:24 Percentile:25.25(Biochemistry & Molecular Biology)Neutron diffraction provides an experimental method of directly locating hydrogen atoms in proteins. High resolution neutron diffractometers dedicated to biological macromolecules (BIX-type diffractometer) have been constructed at the Japan Atomic Energy Research Institute (JAERI) and they have been used in the 1.5 AA -resolution crystal structure analyses of several proteins.

Hua, Y.*; Narumi, Issei; Gao, G.*; Tian, B.*; Sato, Katsuya; Kitayama, Shigeru; Shen, B.*
Biochemical and Biophysical Research Communications, 306(2), p.354 - 360, 2003/06
Times Cited Count:152 Percentile:95.76(Biochemistry & Molecular Biology)We have identified a unique deinococcal gene, 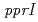 , as a general switch for downstream DNA repair and protection pathways, from a natural mutant, in which
, as a general switch for downstream DNA repair and protection pathways, from a natural mutant, in which 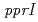 is disrupted by a transposon. Complete functional disruption of the gene in wild-type leads to dramatic sensitivity to ionizing radiation. Radioresistance of the disruptant could be fully restored by complementation with
is disrupted by a transposon. Complete functional disruption of the gene in wild-type leads to dramatic sensitivity to ionizing radiation. Radioresistance of the disruptant could be fully restored by complementation with 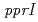 . In response to radiation stress, PprI can significantly and specifically induce the gene expression of
. In response to radiation stress, PprI can significantly and specifically induce the gene expression of 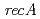 and
and 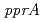 and enhance the enzyme activities of catalases. These results strongly suggest that PprI plays a crucial role in regulating multiple DNA repair and protection pathways in response to radiation stress.
and enhance the enzyme activities of catalases. These results strongly suggest that PprI plays a crucial role in regulating multiple DNA repair and protection pathways in response to radiation stress.
Niimura, Nobuo; Chatake, Toshiyuki; Ostermann, A.; Kurihara, Kazuo; Tanaka, Ichiro
Zeitschrift f r Kristallographie, 218(2), p.96 - 107, 2003/03
r Kristallographie, 218(2), p.96 - 107, 2003/03
no abstracts in English
Yura, Kei; Go, Michiko*
Tampakushitsu Kakusan Koso, 47(8), p.1090 - 1096, 2002/06
no abstracts in English
Makuuchi, Keizo
Porima Daijesuto, 54(2), p.17 - 25, 2002/02
no abstracts in English
Makuuchi, Keizo; Haque, M. H.*; Ikeda, Kenichi*; Yoshii, Fumio; Kume, Tamikazu
Nihon Ratekkusu Arerugi Kenkyukai Kaishi, 4(1), p.7 - 10, 2001/01
no abstracts in English
Takeshita, Hidefumi; Ishida, Kazushige*; Kamiishi, Yoichi*; Yoshii, Fumio; Kume, Tamikazu
Macromolecular Materials and Engineering, 283, p.126 - 131, 2000/11
no abstracts in English
Takeshita, Hidefumi; Ishida, Kazushige*; Kamiishi, Yoichi*; Yoshii, Fumio; Kume, Tamikazu
Shokuhin Shosha, 35(1-2), p.49 - 53, 2000/10
no abstracts in English
Pewlong, W.*; Sudatis, B.*; Takeshita, Hidefumi; Yoshii, Fumio; Kume, Tamikazu
Shokuhin Shosha, 35(1-2), p.54 - 58, 2000/09
no abstracts in English
Rong, L.*; Yamane, T.*; Niimura, Nobuo
Journal of Crystal Growth, 217(1-2), p.161 - 169, 2000/07
Times Cited Count:27 Percentile:84.41(Crystallography)no abstracts in English
Pewlong, W.*; Sudatis, B.*; Takeshita, Hidefumi; Yoshii, Fumio; Kume, Tamikazu
JAERI-Conf 2000-003, p.146 - 152, 2000/03
no abstracts in English
Takeshita, Hidefumi; Ishida, Kazushige*; Kamiishi, Yoichi*; Yoshii, Fumio; Kume, Tamikazu
JAERI-Conf 2000-003, p.139 - 145, 2000/03
no abstracts in English
Makuuchi, Keizo; Upul, R. M.*; Soebianto, Y. S.*; Yoshii, Fumio
Nihon Ratekkusu Arerugi Kenkyukai Kaishi, p.31 - 37, 1999/07
no abstracts in English
Niimura, Nobuo
Hyomen, 37(3), p.50 - 57, 1999/03
no abstracts in English